不孕症解方——
台大研究證實附睪蛋白質協助精子成熟與結構穩定提高受孕成功機率
不孕症長期為全球關注之重要議題,台灣每年花費在治療不孕症相關的生物技術總值更超過新台幣200億。台大獸醫專業學院暨獸醫學研究所蔡沛學助理教授主持的繁殖生理與細胞生物學實驗室近期發表研究成果,證實男性附睪內蛋白質有助精子成熟與結構穩定,進而提高受孕成功機率,為不孕症治療提供新解方。
蔡沛學表示,此研究與國內外實驗室共同合作,結合大規模proteomic screening, whole tissue imaging, epididymosome isolation與glycan analyses,針對附睪內分泌型蛋白質Quiescin Q6 Sulfhydryl Oxidase (QSOX)的調控與功能進行深入研究。蔡沛學指出,此研究首次證實了男性生殖道蛋白硫氫氧化酶 QSOX (quiescin Q6/sulfhydryl oxidases protein) 的兩種亞型 QSOX1、QSOX2,大量分佈於雄性生殖道中,並於精子成熟過程的重要器官-附睪中呈現互補性的分佈(complementary distribution)。實驗室更透過附睪分泌小體(epididymosome) 的純化與蛋白質定性、定量測量,證實QSOX2受到附睪上皮細胞特殊的apical blebbing 分泌機制調控,於分泌後附著於精子頭、頸交接處,對於穩定精子構造與尾部擺動扮演重要的角色。然而另一亞型,QSOX1 則分布於管腔中,並特異性的緊密黏附在精子頭帽處,調控附睪精子,提供附睪內精子去活化,抑制精、卵結合前非特異性頭帽反應的重要保護機制。
蔡沛學也說明,透過與東京大學獸醫系MAEDA教授及MATSUDA教授領導的實驗室合作,以 Kisspeptin 基因轉殖鼠結合 in vitrocell-based assays了解到 QSOX2的基因與蛋白質表現受到睪固酮 (testosterone) 的調控,並可能藉由附睪上皮細胞principal cells 上的非典型雄性激素受體(atypical androgen receptor) 來調控QSOX2 的表現與分泌。此外,實驗室更以此計畫中所建立的QSOX1-eGFP與 QSOX2-eGFP系統,證實另一亞型 QSOX1的表現與分泌並非如 QSOX2單純受到生殖荷爾蒙的調控,而是與管腔中是否有精子的存在相關(即精子為調控 QSOX1分泌的主要因素)。
蔡沛學表示,此一重大發現徹底顛覆了教科書上傳統生殖道上皮細胞以單方向傳遞精、卵結合所需物質給精子的想法,進一步證實精子可能透過分泌細胞訊息傳遞所需物質給生殖道上皮細胞,建立雙向(bi-directional)溝通與互動的機制。
(此研究成果發表於繁殖生理知名國際期刊Biology of Reproduction,以及11月期刊封面:https://www.ncbi.nlm.nih.gov/pubmed/2980009)

圖一:蔡沛學助理教授繁殖生理與細胞生物學實驗室成員(右一:博班學生汪澤恩)。

圖二:蔡沛學助理教授(右)、博班學生汪澤恩(中)與University of Newcastle Prof. Brett Nixon(左)。
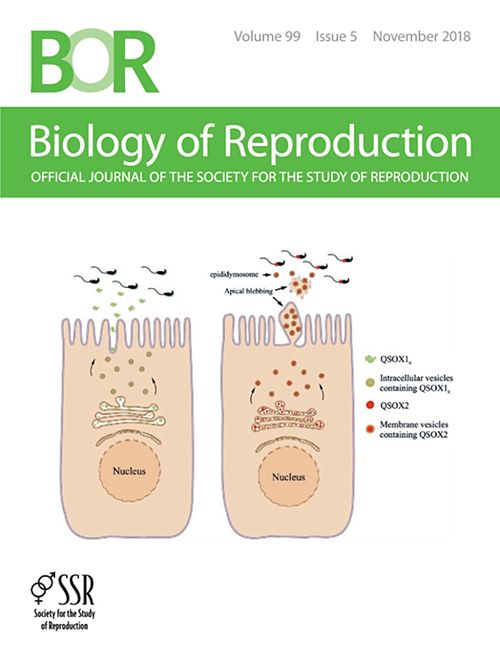
圖三:發表之論文登上繁殖生理知名國際期刊Biology of Reproduction (2018年11月份 cover story。Biology of Reproduction 2018 Nov. 99(5);1022-1033)。
Testicular Protein Assists in Sperm Maturation and Structure Stabilization to Increase the Chance of Pregnancy Success
The Laboratory of Reproductive Physiology and Cell Biology led by Assistant Professor Pei-Shiue Tsai at the School of Veterinary Medicine and Institute of Veterinary Medicine has recently published research results confirming that a testicular protein could help sperm to mature and stabilize their structure, thereby increasing the chance of successful conception and providing new solutions to infertility treatment. Professor Tsai said that this research was done in cooperation with domestic and foreign laboratories, combining large-scale proteomic screening, whole tissue imaging, epididymosome isolation, and glycan analyses to conduct in-depth research on the regulation and function of the testicular endocrine protein Quiescin Q6 Sulfhydryl Oxidase (QSOX). Professor Tsai pointed out that this study confirmed for the first time that the two subtypes QSOX1 and QSOX2 of this protein were distributed in large numbers in the male reproductive tract. By epididymosome purification and qualitative and quantitative measurement of its proteins, Prof. Tsai’s laboratory confirmed that QSOX2 was regulated by the special apical blebbing secretion mechanism of epithelial cells, and it attached to the junction of the sperm head and neck after secretion, affecting sperm structure and tail swing. The other subtype, QSOX1, was distributed in the lumen and adheres specifically to the sperm head cap, which regulated the testicular sperm, induced sperm deactivation, and inhibited the non-specific head cap before sperm and egg binding.
.jpg)
Figure. Professor Pei-Shiue Tsai (right), PhD student Wan (middle), and Prof. Brett Nixon from University of Newcastle (left).